The properties of matter are determined by their molecular structure. Surfactant molecules have an asymmetric amphiphilic structure, that is, the molecular structure of all surfactants contains long non-polar chains, usually long carbon chain hydrocarbons; it can be soluble in oil but not in water. , which is the so-called hydrophobic end or hydrophobic end, and the other end is water-soluble, which is the hydrophilic end. Therefore, surfactant molecules have dual properties of hydrophilic and lipophilic.
First of all, it has the tendency to adsorb on different interfaces in the system, and the surfactant molecules adsorbed on the interface adopt a regular directional arrangement;
Second, they tend to clump together in solution to form bundle-like structures called micelles. Therefore, interfacial adsorption, directional arrangement, and micelle generation are the basic properties of surfactants. Of course, two-parent matchmaking is also an important basic property of it. Figure 1-1 shows the molecular structure of a typical surfactant (soap).

Figure 1-1 Molecular structure of surfactant
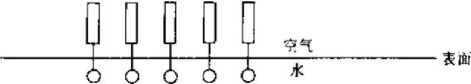
Figure 1-2 Surface orientation of surfactants
The concentration of surfactant that has no significant effect on the properties of the solution is generally in the range of 10-10-‘mol/L and is directly related to the basic properties mentioned above. The effects of surfactants, such as wetting, penetration, emulsification, dispersion, solubilization, foaming, defoaming, and washing, have been widely used in various industries. In addition, in practical applications, there are many basic properties related to surfactants. Indirectly related effects such as smoothing and anti-friction, leveling, color fixation, elimination of static electricity, sterilization, etc.
When surfactant molecules are dissolved in water, both the positive and negative ion parts are solvated by the water. Some of the hydrogen bonds in the water are destroyed by the hydrophobic ends of the surfactant molecules. Interfacial adsorption can keep surfactant molecules in the solution without destroying many hydrogen bonds. Interfacial adsorption means that the hydrophilic end of the surfactant molecule is inserted into the water and is truly dissolved, while the hydrophobic end is neatly arranged in the water. On the surface, the interface orientation is formed, which rarely interferes with hydrogen bonds, and also significantly reduces the surface tension of water. “Figure 1-2.
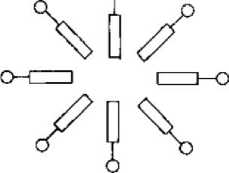
Figure 1-3 Basic structure of micelles
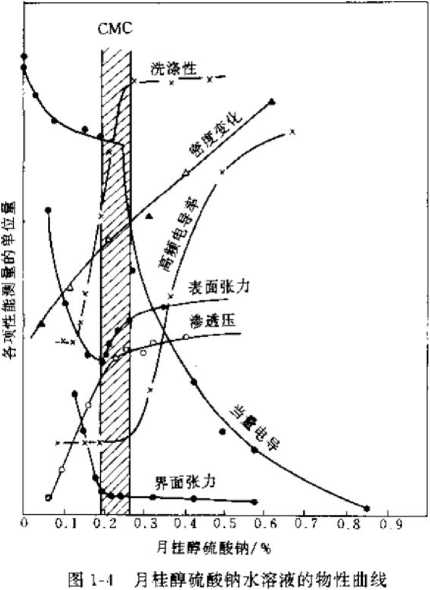
The concentration limit at which a surfactant solute forms micelles in a solution is called the critical micelle concentration (CMC). When the surfactant solute concentration is lower than the CMC, the concentration maintains a linear relationship with the solution characteristics. When it reaches the CMC, it suddenly changes into a curve. Before and after the CMC, the change pattern is very different. As shown in Figure 1-4. Forming micelles is another characteristic of surfactants. The formation of micelles does not require the solute to reach saturation, but below a certain concentration, surfactant molecules will enter the solution in the form of micelles. The basic structure of micelles is shown in Figure 1-3. It is an ion cluster with the hydrophilic end facing out and the hydrophobic end facing inward.
One of the important characteristics of micelles in applications is its solubilization effect. It is generally believed that the interior of micelles has the same state. Therefore, the saturated concentration of the solubilized substance dissolved in a surfactant solution with a concentration above CMC is called the solubilization amount, which is caused by the addition of water-insoluble waistbands inside the surfactant solution with a concentration above CMC.
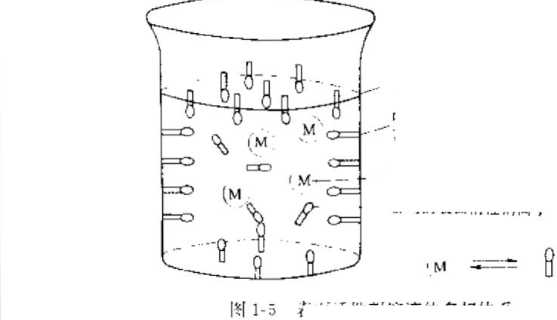
Multiphase equilibrium system of surfactants
Figure 1-5 is a multi-phase equilibrium system of an anionic surfactant solution when it reaches the critical micelle concentration. It can be seen from the figure that at the gas-liquid interface, due to surface adsorption, there are more molecules on the interface than in the solution, resulting in interface orientation. There are also more molecules at the liquid-solid interface (that is, on the walls) than in the solution. Micelles with hydrophilic ends facing outward also appeared in the solution. The molecules in the micelles were in a dynamic guard state because their concentration was higher than that of the molecules in the solution.
SurfaceSex agent application
Wash
The washing function is the most important function of surfactants. The largest consumption sectors of various industrially produced surfactants are household laundry detergents, liquid detergents and industrial cleaning agent. During the application process, the specific embodiment of the washing function is to wash away dirt from various solid surfaces. According to the viewpoint of modern surface-active chemistry, the definition of dirt should be a substance in the wrong position, and removing dirt means doing work. For several thousand years, humans have always had to wash clothes in their daily lives. In the past, it mainly relied on manual labor. Modern washing machines and energy-saving requirements mainly rely on synthetic detergents compounded with various high-efficiency surfactants and other chemicals to complete laundry. .

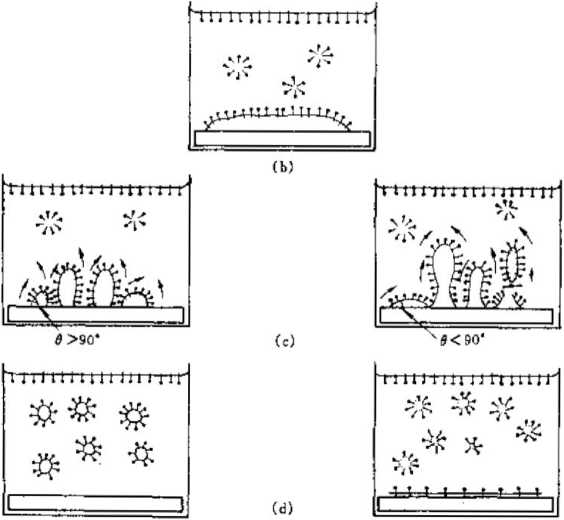
Figure 1-6 “Dissolution effect” of surfactants in the washing process
Figure 1-6 describes a typical washing process for removing oil stains from the fabric surface.
Moisturizing
When a solid surface comes into contact with a liquid, the original gas-solid interface disappears and a new liquid-solid interface is formed. This phenomenon is called wetting. Water can form oxygen. bonds and therefore have high surface tension. When water drops fall onto the surface of a new fabric, it has a certain degree of hydrophobicity because the fabric is usually post-processed. The high surface tension of water causes it to form water droplets and remain on the fabric. If a small amount of surfactant is added to the water, the surface tension of the water can be significantly reduced. The water droplets spread quickly to achieve complete wetting.
The principle of wetting can be regarded as the interface orientation effect of a surfactant. The hydrophobic end of the surfactant is inserted into the surface of the fabric to form an interface orientation, while the hydrophilic end remains in the boundary layer of water, causing the water droplets to stretch into a plane.
If an oil-containing fabric is immersed in a surfactant solution, the surfactant molecules will immediately be adsorbed by the fabric. These molecules will squeeze into the oil film and The oil film is divided into small droplets and can be rinsed off. At this time, the surface of the fabric is no longer wetted by oil, but by a solution of surfactant molecules. This effect is called rewetting.
Emulsification
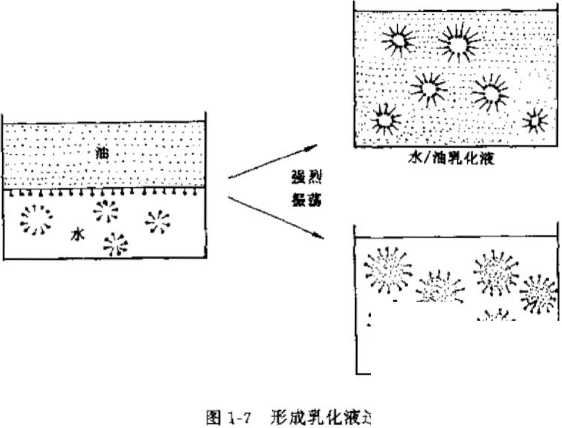
Emulsification is a liquid-liquid interface phenomenon. When two mutually insoluble liquids, such as oil, are added to water, they will naturally form two layers in the container: oil on the upper layer and water on the other. On the lower level. If a suitable surfactant is added, the oil layer will be dispersed under strong stirring, and the hydrophobic end of the surfactant will be adsorbed to the interface layer of the oil beads to form a uniform fine droplet emulsion. This process is called emulsification. See Figure 1-7.
Flotation
The flotation situation is more complicated. It involves at least three phases: gas, liquid and solid. First, a surfactant that can generate large amounts of foam (i.e., a foaming agent) is used. When air is introduced into the water or air enters the water due to the agitation of the water, the hydrophobic end of the surfactant will move toward the bubbles at the air-liquid interface. The air is oriented in one direction. The hydrophilic end is still in the solution, forming bubbles. Another kind of surfactant that plays a trapping role is adsorbed on the surface of solid mineral powder. This adsorption has certain selectivity depending on the mineral. And outward
(a) The oil stain begins to come into contact with the surface active application solution,
(b) The hydrophobic end of the surfactant dissolves into the grease,
(c) Surfactants affect the connection between oil dirt and fabrics. Figure 1-6 The “dissolution effect” of surfactants during the washing process. Min seeks the contact angle e>9. In this way, a water vortex will be formed under the strong agitation of the washing machine, so that the oil stains on the fabric will be washed off.
(d) Further mechanical force will turn the oil into a suspension and be washed away. The hydrophobic end of the liquid will be partially inserted into the bubble. In this way, the bubbles can take away the designated mineral powder during the flotation process to achieve the purpose of mineral processing. Figure 1-8 Schematic diagram of the flotation process. In practical applications, the amount of surfactant used is very small, and generally 10 ghosts of capture are used. The agent can handle the slurry of 3r water and It mineral powder.

 微信扫一扫打赏
微信扫一扫打赏

