Background and overview[1]
4-Hydroxy-3-methoxy-2-nitrobenzaldehyde can be used as a pharmaceutical intermediate, which can be prepared through a three-step reaction using vanillin as the starting material. There are reports in the literature that 4-hydroxy-3-methoxy-2-nitrobenzaldehyde can be used to prepare a new type of macrocyclic heterocyclic compound with an 18-19-membered heterocycle as the core that is different from the existing structure. This university Cyclic heterocyclic compounds have better inhibitory activity against hepatitis C virus, can be effectively used to treat hepatitis C virus infection, and have low toxic and side effects.
Preparation[1]
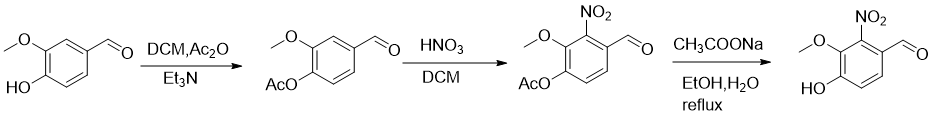
1) Vanillin (10g, 65.8mmol), triethylamine (13.3g, 136mmol) and DMAP (0.1g) were added to DCM (100mL), then acetic anhydride (8g, 79mmol) was added, and the reaction was carried out at room temperature 2h, after TLC and LC-MS detection, the reaction was completed, the system was washed three times with 2N hydrochloric acid, the organic phase was dried and concentrated to obtain a crude product, which was purified through column to obtain 12g of white solid, with a yield of 94%. 1HNMR (300MHz, CDCl3, ppm): δ9.96(s,1H),7.51-7.48(m,2H),7.88-7.22(m,1H) ),3.92(s,3H),2.36(s,3H).
2) Add fuming nitric acid (10g, 155mmol) to DCM (200mL), cool to -12 degrees, then add acetyl-protected vanillin (20g, 103mmol) in batches, and follow the reaction with TLC until completion. Slowly pour the reaction solution into ice water, separate the layers, extract the aqueous phase three times with DCM, combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, concentrate to obtain the crude product, and beat with diethyl ether to obtain the product (1.4g, 57% ).1HNMR (300MHz, CDCl3, ppm): δ9.92 (s, 1H), 7.72 (d, J = 8.4Hz, 1H), 7.46 ( d,J=8.4Hz,1H),3.97(s,3H),2.42(s,3H).
3) The nitration product (10g, 42mmol) was added to ethanol (100mL), and then an aqueous solution (50mL) of sodium acetate (6.85g, 83.5mmol) was added dropwise. After the dripping was completed, heated to reflux, TLC, LC-MS Check that the reaction is complete, add water, extract with EA, wash with brine, dry, and concentrate to obtain crude 4-hydroxy-3-methoxy-2-nitrobenzaldehyde. 1HNMR (300MHz, DMSO-d6, ppm): δ9.72(s,1H),7.71(d,J=8.7Hz,1H),7.21(d,J=8.4Hz,1H ),3.84(s,3H).
References
[1]CN201780000221.0 A class of macrocyclic heterocyclic compounds that inhibit hepatitis C virus and their preparation and use

 微信扫一扫打赏
微信扫一扫打赏

