Background and overview[1-2]
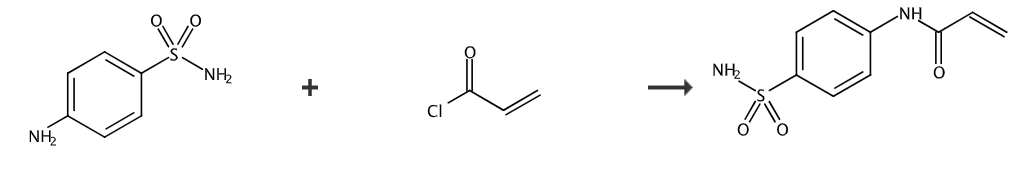
N-[4-(Sulfonamide)phenyl]acrylamide (ASPAA) monomer can be homopolymerized or copolymerized with other monomers to form pharmacologically active polymers that are water-soluble, have little toxic and side effects, and have mitigating properties. Large molecule drugs that release effects. At the same time, because the sulfonamide group has certain alkali solubility, copolymers of ASPAA and acrylic esters and other monomers can be used to prepare lithographic and computer-to-plate printing plates with good properties such as alkali developability, development latitude, and print durability.
Preparation[1][3]
Report 1,
Add sulfonamide (1.00g) and pyridine (1.41mL) to 1,4-dioxane (30mL). Acryloyl chloride (0.49 mL) was added dropwise and the solution was stirred at room temperature for 3 hours. 1M HCl was added and the solution was extracted with ethyl acetate. The extract was dried over brine and anhydrous sodium sulfate, and the solvent was removed to give a crude product that was pure enough for subsequent use.
Report 2,
Add 13.76g (80mmol) of p-aminobenzenesulfonamide, a certain amount of NaHCO3, 20mg of 1,3-dinitrobenzene, and 100mL of DMF into a dry three-necked flask. Use ice for the reaction solution Cool the bath to 0°C to 2°C, slowly add 7.96g (88mmol) of acryloyl chloride through a constant pressure dropping funnel while stirring, and react at a certain temperature for 3 hours after the dropwise addition is completed. Filter to remove the salt, pour the filtrate into 10 times the volume of methanol-water, and precipitate a white solid. Filter, wash the filter cake with water 3 to 5 times, and then recrystallize it with methanol to obtain ASPAA. The yield is 50% to 65%, m.p. 234℃~ 236℃;1HNMRδ: 10.5 (s, 1H, CONH), 7.83~7.85 (d, 2H, ArH), 7.79~7.80 (d, 2H, ArH), 7.29 (s, 2H, NH2), 5.81, 6.33 (m, 2H, =CH2), 6.47 (t, 1H, =CH); IR ν: 3342 (N-H), 3133, 3066 (=C-H), 1676 (C=O, amide I spectrum band), 1536 (CONH, amide II band), 1593, 1493 (-C=CH), 1411 (C-N stretching vibration), 1164 (SO2-N), 836 (benzene ring disubstituted) cm-1 ;Ana.lcalcd for C9H10N2O3S:C47.78, H4.45, N12.38; found C 47.12, H4.33, N12.21
References
[1]FromPCTInt.Appl., 2010065865, 10Jun2010
[2] Gao Yingxin, Bao Yongzhong, Huang Zhiming, Weng Zhixue. Reactivity rate of copolymerization of N-[4-(sulfonamide)phenyl]acrylamide with acrylonitrile and methyl methacrylate[J]. High Acta Molecule, 2004(03):450-453.
[3] Gao Yingxin, Bao Yongzhong, Huang Zhiming, Weng Zhixue. Synthesis of N-[4-(sulfonamide)phenyl](meth)acrylamide [J]. Synthetic Chemistry, 2005(01):22- 24+36-1.

 微信扫一扫打赏
微信扫一扫打赏

