Background and overview[1]
Biphenyl polyimide, as a high-performance polymer, has heat resistance, solvent resistance, radiation resistance, and good mechanical and dielectric properties. It is widely used in mechanical electronics, aerospace, large motors, turbine bearings, etc. Heat-resistant filter materials and other fields have broad application prospects. Compared with the polyimide in which 3,3′,4,4′-biphenyltetracarboxylic dianhydride (4,4′-BPDA) is a monomer with a symmetrical structure, the asymmetric structural isomer 2,3,3 ‘,4′-Biphenyltetracarboxylic dianhydride (3,4′-BPDA) is a monomer polyimide with higher Tg and lower melt viscosity, which significantly improves the processability during material preparation. sex. Therefore, the use of 3,4′-BPDA to replace 4,4’-BPDA matrix resin has received more and more attention.
Preparation[1]
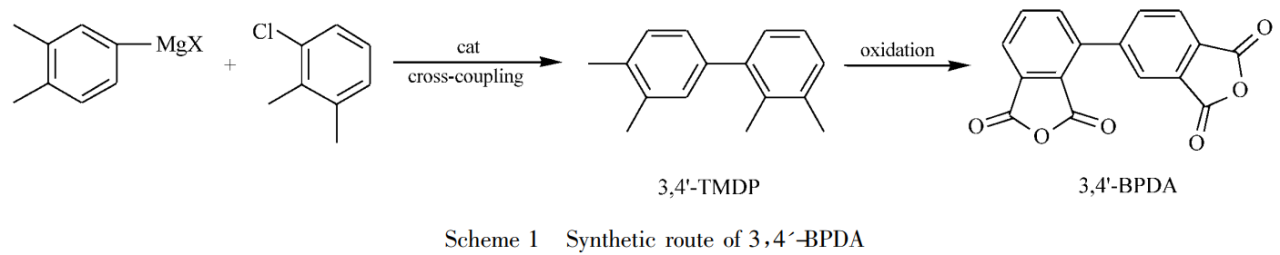
Li Yuhan et al. reported a method for efficiently preparing 3,4′-biphenyltetracarboxylic dianhydride (3,4′-BPDA) using halogenated o-xylene as raw material through cross-coupling, oxidation and other processes. method. As shown in Schmeme1, 4-halo-o-xylene reacts with Mg to prepare Grignard reagent, and then cross-coupling is performed under transition metal catalysis to prepare 2,3,3’4′-tetramethylbiphenyl (3,4′ -TMDP). Finally, the target compound 3,4′-biphenyltetracarboxylic dianhydride (3,4′-BPDA) was obtained by transition metal catalyzed oxidation.
1. Preparation of tetramethylbiphenyl from mixed chlorinated o-xylene
Into a 500mL single-neck flask, add mixed chlorinated o-xylene (112g, 0.80mol), tetrahydrofuran (THF, 16mL, 0.20mmol), magnesium chips (3.88g, 0.16mol) and a small amount of I2 (0.02g, 0.08mmol), stir and mix. Heat to 110°C under N2 gas protection, and react until all the magnesium chips disappear (about 8 hours). The reaction mixture was cooled to room temperature, TMEDA (18 mL, 0.12 mol) and Ni(acac)2 (0.018 g, 0.070 mmol, acac: acetylacetone) were added, and stirring was continued. The reaction system was slowly raised to 110°C and reacted for 6 hours. After the reaction is completed, cool to room temperature, add 500 mL of hydrochloric acid solution (1 mol/L) to the reaction mixture, separate the layers, and extract the aqueous layer with dichloromethane (100 mL × 2). The organic layers were combined and dried over anhydrous magnesium sulfate. The magnesium sulfate was filtered off and the solvent was evaporated to dryness to obtain a crude product. The crude product was analyzed by gas chromatography. The conversion rate is 81%, and the ratio of the three isomers is n(3,3′-TMDP):n(3,4′-TMDP):n(4,4′-TMDP)=1:2.7:2.
2. Preparation of 3,4′-tetramethylbiphenyl (3,4′-TMDP) from single halogenated o-xylene
Under N2 gas protection, add magnesium chips (about 0.290g, 12.0mmol), 3,4-dimethylbromobenzene (1.85g, 10.0mmol), and anhydrous tetrahydrofuran (8mL) into a 50mL three-necked flask. Under stirring conditions, isopropyl magnesium chloride (1 mol/L, 0.5 mL) was added dropwise, and then heated to initiate the reaction, so that the reaction was in a slightly boiling state. When no obvious bubbles are generated in the reaction system (about 1 hour), heat and reflux for another 1 hour to prepare 3,4-xylylmagnesium halide. Add NiF2 (0.067g, 0.70mmol), triphenylphosphine (PPh3, 0.204g, 0.72mmol) and 2mL anhydrous tetrahydrofuran to a 25mL three-necked flask. And stir for 30 minutes to prepare the catalyst. Under the protection of N2 gas, 2,3-dimethylchlorobenzene (0.984g, 7.00mmol) and the 3,4-dimethylphenylmagnesium halide prepared above were added to the catalytic system. Reflux reaction for 8 hours. After stopping the reaction, pour the reaction solution into 10 mL of saturated ammonium chloride solution, separate the organic phase, and extract the aqueous phase with ethyl acetate. The organic phases were combined, dried over anhydrous magnesium sulfate, and the solvent was evaporated. A crude product of 2,3,3′,4′-tetramethylbiphenyl (3,4′-TMDP) was obtained. The obtained crude product of 3,4′-TMDP was analyzed by gas chromatography, and the yield was 93%. 1HNMR (DMSO-d6, 300MHz), δ: 7.21 (d, 1H, J=7.2Hz), 7.18 (s, 1H), 7.17 (d, 1H, J=7.2Hz), 7.12 (dd, 2H, J=7.2, 0.3Hz), 7.0 (dd, 1H, J=7.2, 0.3Hz).
3. Oxidation of 2,3,3′,4′-tetramethylbiphenyl to prepare 2,3,3′,4′-biphenyltetracarboxylic dianhydride
Add 3,4′-TMDP (2.10g, 10.0mmol), cobalt acetate (0.017g, 0.10mmol), sodium bromide (0.027g, 0.25mmol) and 15mL acetic acid to the stainless steel pressure reactor. Add N2-O2 mixed gas (partial pressure ratio = 2:1) into the reaction bottle to keep the pressure at 0.5MPa. Then, the reaction system was heated until the internal temperature reached 100°C, and the reaction was carried out for 6 hours. After the reaction, the temperature of the reactor dropped to room temperature, and then the reaction mixture was poured into 50 mL of water and left to stand overnight. A large amount of white solid was formed. The white solid is filtered and washed with water, and the obtained filter cake is vacuum-dried at 60°C to obtain 2,3,3′,4′-biphenyltetracarboxylic acid as a white powder. Finally, biphenyltetracarboxylic acid was heated to 250°C for melting and dehydration for 12 hours to obtain 2.40g of white crystals, which is 2,3,3′,4′-biphenyltetracarboxylic acid dianhydride. The yield was 80%, mp197~199°C . 1HNMR (DMSO-d6, 600MHz), δ: 8.41 (s, 1H), 8.27 (dd, 1H, J=7.2, 1.2Hz), 8.23 (d, 1H, J=7.8Hz ), 8.18 (d, 1H, J=7.2Hz), 8.11 (t, 1H, J=7.2Hz), 8.09 (dd, 1H, J=7.2, 1.2Hz),
Apply[2-3]
Application 1,
CN201510589813.3 reports a method for preparing a low thermal expansion coefficient polyimide film, which includes the following steps: (1) placing pyromellitic dianhydride and p-phenylenediamine in a dimethylacetamide solvent for polycondensation Reaction to obtain polyamic acid glue A; (2) Combine 2,3′,3,4′-biphenyltetracarboxylic dianhydride and 4,4′-diamino acidDiphenyl ether is placed in a dimethylacetamide solvent to perform a polycondensation reaction to obtain polyamic acid glue B; (3) Mix polyamic acid glue A and polyamic acid glue B, and stir at high speed to obtain polyamic acid. Glue solution C; (4) The polyamic acid solution C is made into a film through a casting process, and finally the film is sent to an imidization furnace for processing, thereby obtaining the low thermal expansion coefficient polyimide film. The low thermal expansion coefficient polyimide film produced by the present invention has a CTE of 15 to 17 ppm/K, and also has performance advantages such as high strength, high stability, and high electrical strength.
Application 2,
CN201210115662.4 discloses a production method of light-colored transparent polyimide film, which includes the following production steps: (1) fluorine-containing aromatic diamine 1,4-bis(4′-amino-2′ -Trifluoromethylphenoxy)biphenyl (TFDAB) is dissolved in the polar aprotic solvent N,N-dimethylacetamide under stirring; (2) 2,3,3′,4′-dimethylacetamide Pyromellitic dianhydride (α-BPDA) is added to the completely dissolved fluorine-containing aromatic diamine for polycondensation reaction, 2,3,3′,4′-biphenyltetracarboxylic dianhydride and fluorine-containing aromatic diamine 1 , 4-Bis(4′-amino-2′-trifluoromethylphenoxy)biphenyl reacts to obtain a polyamic acid solution; (3) The above polyamic acid solution is thermally imidized on a casting machine, A light-colored and transparent polyimide film was produced. The clear polyimide film produced by the invention has a short production process and good light-colored and transparent effect.
References
[1]Li Yuhan, Wu Qiang, Kang Chuanqing, Guo Haiquan, Jin Rizhe, Gao Lianxun. Synthesis of 2,3,3′,4′-biphenyltetracarboxylic dianhydride [J]. Applied Chemistry, 2016, 33(08 ):900-904.
[2] CN201510589813.3 Preparation method of low thermal expansion coefficient polyimide film
[3] CN201210115662.4 A production method of light-colored transparent polyimide film

 微信扫一扫打赏
微信扫一扫打赏

