Background and overview[1][2]
Methyl 3-methyl-4-nitrobenzoate is a pharmaceutical intermediate that can be obtained by one-step esterification of 3-methyl-4-nitrobenzoic acid. It is the starting material for preparing the antihypertensive drug Telmisartan.
Preparation[1-2]
Report 1,
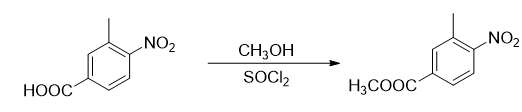
Add 300g 3-methyl-4-nitrobenzoic acid (1.66mol) and 1000mL methanol into a dry 3L three-necked flask equipped with a reflux condenser and a thermometer, and make a simple exhaust gas absorption device on the reflux condenser. The absorption liquid is NaOH aqueous solution. Under electromagnetic stirring, add 100g of thionyl chloride (0.85 mol) drop by drop using a constant pressure dropping funnel. Control the temperature of the reaction solution during the dropping process to be between 30-40°C. At this time, the reaction solution will be a milky white solution. After about 45 minutes, the dropwise addition is completed, and the temperature is raised to 60°C. At this time, the reactant becomes a yellow clear and transparent solution. Stir and reflux the reaction for 2 hours at this temperature, stop heating, and slowly cool the reaction solution. When it reaches 50°C, a large amount of solid precipitates. Continue to cool down to 30℃, add 1000mL water to the reaction solution, stir for about half an hour until no more gas is released, filter the reactant, wash the obtained filter cake with 100mL×3 water, and dry it at 40℃ to obtain 314g of yellow powder. Solid, yield 97.2%, m.p. 80~82°C.
Report 2,
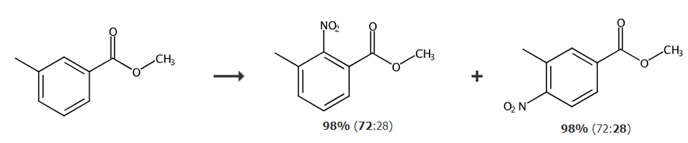
Add 27.2g of 3-methylbenzoic acid, 50mL of methanol, and 4mL of concentrated sulfuric acid into a 250mL single-neck bottle, and conduct a reflux reaction for 2h to 3h. After the reaction, the methanol was concentrated to remove, and the organic phase was washed with saturated potassium bicarbonate solution (30 mL × 3), purified water (30 mL), and dried to obtain 28.82 g of yellow liquid, with a yield of 96.08% and a purity of 98.64%.
Add 4.8mL 65% nitric acid (0.07moL) into a 100mL round-bottomed flask, add 7.6mL (0.08moL) acetic anhydride dropwise, and finish the dripping. After 0.5h reaction, add 7.6mL acetic anhydride, 5.4mL acetic acid, 3.0g3 -After mixing, methyl methyl benzoate (0.02moL) was slowly added dropwise to the reaction system. The above operations were all performed under ice bath conditions. After the dripping is completed, raise the temperature to 30°C and react for 3 hours. After stopping the reaction, evaporate acetic anhydride, nitric acid and the generated glacial acetic acid. Pour the concentrated solution into ice water and stir. A yellow solid will precipitate. Add 20mL of ethyl acetate to adhere to the solution. The product on the wall of the bottle was dissolved, separated and concentrated to obtain 3.83g of light yellow liquid with a yield of 98.21%. After HPLC analysis, the content ratio of methyl 3-methyl-2-nitrobenzoate to methyl 3-methyl-4-nitrobenzoate was 72:28.
Main reference materials
[1][Invented in China] Improvement of the preparation process of CN201310270872.5 telmisartan
[2] [Chinese invention] CN201610933001.0 A method for preparing highly selective 3-methyl-2-nitrobenzoic acid

 微信扫一扫打赏
微信扫一扫打赏

