Background and overview[1]
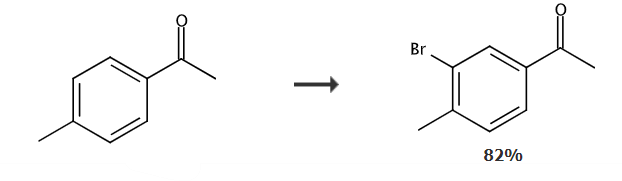
3′-Bromo-4′-methylacetophenone is a bromide of acetophenone derivatives. This type of compound is a very important chemical intermediate in medicines, pesticides, dyes, and spices. It has a wide range of applications in other industries, and it can further prepare various target compounds through Suzuki coupling reaction.
Preparation[1-2]
Report 1,
Put 40.05g (0.24mol) potassium bromate and 100ml water into a 500ml four-neck bottle, and then put the mixed solution of 26.84g (0.2mol) p-methylacetophenone and 90g methanol into the 500ml four-neck bottle. 312g of sodium bisulfite solution with a mass percentage concentration of 20% was added dropwise under stirring conditions. Control the dripping temperature to not exceed 55°C, the dripping time is about 3 hours, and the insulation reaction is 2 hours after the dripping is completed. Cool to room temperature, filter with suction, wash with water, and recrystallize with petroleum ether to obtain 34.95g of light brown solid 3′-bromo-4′-methylacetophenone, yield 82%, melting point (m.p.) = 42~46°C.
Report 2,
Add a solution of 97g (723mol) 4-methylacetophenone in 100ml methylene chloride to 237g (1.78mol) AlCl3In a suspension in 700 ml of methylene chloride. The reaction mixture was then stirred at this temperature for 10 min, and then 40.0 ml (125 g, 0.781 mol, 8% excess) of bromine was added dropwise over 1 h. The resulting mixture was stirred at room temperature overnight and then poured onto 2000 cm3 of ice. The organic layer was separated, and the aqueous layer was extracted with 3x150ml methylene chloride. The combined organic extracts were washed with aqueous K2CO3, dried with K2CO3, and passed through Short pads of silica gel 60 (40-63μm) are then evaporated to dryness. The crude product was distilled under vacuum to yield 143 g (93%) of a colorless liquid (boiling point 125-132°C/8 mm Hg), which solidified completely on standing at room temperature. Anal. Calculated for C9H9BrO: C, 50.73; H, 4.26. Found: C, 50.89; H, 4.12. 1H NMR (CDCl3): δ8.09(s,1H,2-H),7.77(d,J=7.8Hz,1H,6-H) ,7.31(d,J=7.8Hz, 1H,5-H),2.56(s,3H,COMe),2.44(s,3H,4-Me).
Main reference materials
[1] [Chinese invention] CN201910048412.5 Synthesis method of aromatic ring bromination of acetophenone derivatives
[2] [China invention, China invention authorization] CN201380067625.3 Catalyst

 微信扫一扫打赏
微信扫一扫打赏

