Overview[1][2]
4-Cyanomethyl-2,3,5,6-tetrafluorobenzonitrile is a photochemical intermediate that can be prepared from hexafluorobenzonitrile in one step and can be used to synthesize luminescent materials.
Preparation[1]

Add hexafluorobenzonitrile (1.93g, 10mmol), ethyl cyanoacetate (1.11g, 10mmol), potassium carbonate (1.67g, 12.1mmol), and 30mL of DMF into the reactor, and stir for 48 hours at room temperature. Distilled water and acetic acid were added to terminate the reaction, extracted with dichloromethane and concentrated to obtain 4-cyanomethyl-2,3,5,6-tetrafluorobenzonitrile (2.83g, 99%).
Apply[2]
CN201710991310.8 reported that 4-cyanomethyl-2,3,5,6-tetrafluorobenzonitrile can be used to prepare a compound 1 containing a tetrathiafulvalene structure. Tetrathiafulvalene is an organic material with good charge transport properties, especially when it forms free radical molecules. The present invention reduces the triplet singlet energy level difference of the molecule through reasonable design, reasonably adjusts the molecular energy level, and obtains a molecular structure of a tetrathiafulvalene derivative that is conducive to the formation of stable free radicals, and can be used to synthesize new sulfur-containing organic electrolytes. Luminescent material.
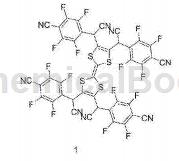
Dissolve calcium acetate (2.8mol) in 100ml of water, add 4-cyanomethyl-2,3,5,6-tetrafluorobenzonitrile (1.2mmol) to the reaction flask, and add acetic acid. After fully dissolving, add 2.4 mmol of sulfuric acid, heat the reaction system to reflux, and react for 6 hours. After the reaction, cool to room temperature, add ice water, separate the organic layer, extract the aqueous layer twice with toluene and combine the organic layers. The organic layer was filtered to remove impurities, and the filtrate was washed three times with water and concentrated at 60°C. Then add 2-propanol and water and let it stand for 1 hour at 0 degrees to obtain precipitation. After filtration, the precipitate was washed three times with a mixed solution of 2 propanol and water, and dried to obtain product 1-C.
Under an inert atmosphere, mix 1 mmol of lithium hydride and 8 ml of anhydrous tetrahydrofuran. Under nitrogen protection, cool the temperature to about 10 degrees. Dissolve 0.4 mmol of the product 1-C in 5 ml of anhydrous tetrahydrofuran and add it dropwise. And keep the system temperature not exceeding 10 degrees. After the addition was completed, the reaction was stirred for 1 hour, and a tetrahydrofuran solution of 0.1 mmol of tetrachlorotetrathiafulvalene was added dropwise. After the addition is completed, stir the reaction for 24 hours, add 10 ml of water to terminate the reaction, and add 2 ml of hydrochloric acid to neutralize the system. Ethyl acetate was added to extract the organic phase. The organic phase was separated and the solvent was evaporated to obtain compound 1 with a yield of 55%.
Main reference materials
[1] [Chinese invention] CN201811118231.7 A fluorene derivative and its organic electroluminescent device
[2] [Chinese invention] CN201710991310.8 A compound containing a tetrathiafulvalene structure and an organic electroluminescent device

 微信扫一扫打赏
微信扫一扫打赏

