Background and overview[1]
3-phenylisoxazole-5-carboxylic acid can be used as a pharmaceutical synthesis intermediate.
Preparation[1]
The preparation of 3-phenylisoxazole-5-carboxylic acid is divided into the following steps:
Step 1: 5-(4-fluorophenyl)-N-(2-morpholinoethyl)isoxazole-3-carboxamide

A stirred solution of 5-(4-fluorophenyl)isoxazole-3-carboxylic acid (0.1g, 0.48mmol) and 2-morpholinoethyl-1-amine (0.127g, 0.97mmol) The dichloromethane (5 mL) solution was cooled to 0 °C. Pyridine (0.2 mL) was added and the reaction was stirred for 10 minutes. POCl 3 (0.2 mL) was added dropwise and the reaction mixture was stirred at 25 °C for 1 h. Ice water was added and the product was extracted into dichloromethane (2 x 25 ml). The organic layer was washed with saturated NaHCO3 (25 ml), brine, dried over Na2SO4 and concentrated to give the crude product, which was purified by flash column chromatography (0-20% ethyl acetate in hexane) to give 5-(4-fluorophenyl)-N-(2-morpholinoethyl)isoxazole-3-carboxamide (0.090 g, 319 [M + H]). 1H MR: (400MHz, DMSO) δ: 2.420 (s, 4H), 2.462-2.479 (m, 2H), 3.374-3.423 (m, 2H), 3.563-3.585 (t, J = 4.4, 4H), 7.365 ( s, 1H), 7.397-7.442 (t, J = 8.8, 2H), 7.998-8.033 (m, 2H), 8.671-8.699 (t, J = 5.6, 1H).
Step 2): ethyl 2,4-dioxo-4-phenylbutyrate

To a stirred solution of NaH (60%) (17.0g, 418mmol) in toluene (400mL) was added dropwise acetophenone (25.0g, 208mmol) at 0°C. 0℃. The reaction mixture was then stirred at 0°C for 30 minutes. Then diethyl oxalate (43 mL, 312 mmol) was added dropwise at 0°C. The reaction mixture was stirred at 25°C for 2 hours. The reaction mixture was diluted with water (1000 mL) and extracted with ethyl acetate (3 x 250 mL). The organic layer was washed with brine (250 mL), dried over anhydrous sodium sulfate and distilled to give crude ethyl 2,4-dioxo-4-phenylbutyrate (48.0 g, 221 [M+H]), For liquid. The material was used in the next step without further purification.
3) 3-phenylisoxazole-5-carboxylic acid
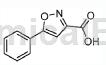
To a stirred solution of ethyl 2,4-dioxo-4-phenylbutyrate (48.0g, 218mmol) in ethanol (600mL) at 0°C was added hydroxylamine hydrochloride (46.0g, 654mmol) Part wise. The mixture was then stirred at 80°C for 3 hours. The reaction mixture was concentrated under reduced pressure, and the resulting residue was suspended in water (500 mL). The precipitate was collected by filtration and dried under vacuum to give the crude product, which was purified by column chromatography (0-7% ethyl acetate in hexane) to give 3-phenylisoxazole-5-carboxylic acid (28.0 g, 218 [M + H]).
Main reference materials
[1] WO2016054560) ISOXAZOLE COMPOUNDS AND METHODS FOR THE TREATMENT OF CYSTIC FIBROSIS

 微信扫一扫打赏
微信扫一扫打赏

