Background and overview[1]
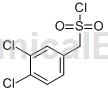
(3,4-Dichlorophenyl)methanesulfonyl chloride is an aromatic hydrocarbon derivative that can be used as a pharmaceutical synthesis intermediate.
Structure
Preparation method[1]
The preparation of (3,4-dichlorophenyl)methanesulfonyl chloride is divided into the following steps:
Step 1: Preparation of 3,4-dichlorophenylmethanesulfonic acid sodium salt
To a suspension of 3,4-dichlorobenzyl chloride (0.68 mL, 5.0 mmol) in 20 mL of water was added sodium sulfite (630 mg, 5.0 mmol), and the mixture was heated to reflux overnight. The reaction mixture was thoroughly evaporated using a rotary evaporator and dried under vacuum to give 3,4-dichlorophenylmethanesulfonic acid sodium salt as a white solid.
1H NMR (400MHz, DMSO-D6) δppm 3.97 (s, 2H) 7.28 (t, J=7.83Hz, 1H) 7.49 (m, 2H)
Step 2: Preparation of 3,4-dichlorophenylmethanesulfonic acid
Suspend 3,4-dichlorophenylmethanesulfonic acid sodium salt (5.0 mmol) in 50 mL MeOH, stir at 50°C for 1 hour, and then cool to -10°C. HCl gas was bubbled in for a few seconds and the resulting white suspension was stirred at -10°C for 1 hour. The mixture was filtered through Celite(R) and evaporated. The obtained residue was triturated with 60 mL of anhydrous acetone and filtered. The filtrate was evaporated to give a yellow semi-solid. This solid was triturated with 40 mL of 2:1 ether:hexane. The solid obtained was filtered and washed with hexane to give 902 mg of 3,4-dichlorophenylmethanesulfonic acid.
1H NMR (400MHz, DMSO-D6) δppm 3.96 (s, 2H) 7.27 (t, J = 7.83Hz, 1H) 7.49 (m, 2H)
Step 3 3,4-Dichlorophenylmethanesulfonyl chloride
Dissolve 3,4-dichlorophenylmethanesulfonic acid (260 mg, 1.0 mmol) in 5 mL of anhydrous THF and cool to 0°C. A catalytic drop of DMF was added, followed by oxalyl chloride (0.44 mL, 5.0 mmol). The reaction mixture was allowed to warm to room temperature for 90 minutes and then filtered through Celite(R), rinsing the Celite(R) with an additional 15 mL of anhydrous THF. The filtrate was evaporated to a volume of approximately 5 mL, then 5 mL of water was added in small portions and the vessel was cooled in a water bath. The mixture was extracted with 2×25 mL EtOAc, and the combined organic phases were washed with saturated sodium bicarbonate, brine, and dried (MgSO4). Filtration and evaporation gave the crude product as a yellow oil. Silica gel chromatography using a gradient from 5% EtOAc/hexane to 30% EtOAc/hexane gave 142 mg of (3,4-dichlorophenyl)methanesulfonyl chloride as a white solid.
1H NMR (400MHz, CHLOROFORM-D) δppm 5.09 (s, 2H) 7.25 (t, J=7.96Hz, 1H) 7.45 (m, 1H) 7.53 (dd, J=8.08, 1.52Hz, 1H)
Main reference materials
[1] (CN1922136) Preparation method of aryl- and heteroaryl-alkylsulfonyl halides

 微信扫一扫打赏
微信扫一扫打赏

