Background and overview[1-2]
Calcium dobesilate was put into clinical use in the 1970s for the prevention and treatment of diabetic retinopathy. Calcium dobesilate (monohydrate) is a hydrate formed by the reaction between calcium dobesilate and water. This drug mainly reduces capillary permeability, inhibits platelet aggregation reaction, and reduces blood viscosity. Calcium dobesilate capsule is an oral vasoprotective agent that has significant inhibitory and reversal effects on the “three highs” factors of diabetic retinopathy (DR). It can reduce the high permeability of capillaries, reduce the high viscosity of blood, and reduce the high activity of platelets, thereby reducing retinal exudation and bleeding, reducing microaneurysms, etc. Studies have shown that starting preventive medication before clinically visible retinopathy occurs after diabetes is diagnosed has a better prognosis for DR.
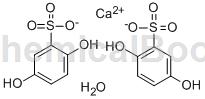
Structure
Purpose[3]
This product regulates the physiological functions of the microvessel wall, reduces resistance, reduces the permeability of the microvessel wall, increases its resistance, reduces blood and plasma viscosity, corrects the albumin/globulin ratio, reduces platelet hyperaggregation, and thereby It can prevent thrombosis and improve the flexibility of red blood cells; it can indirectly increase lymphatic drainage and reduce edema. It can also inhibit the hyperosmotic effect of vasoactive substances (histamine, 5-hydroxytryptamine, bradykinin, hyaluronidase, prostaglandins) on microvessels, improve the biosynthesis of basement membrane collagen, and thereby protect microvessels.
Pharmacokinetics[3]
After oral administration of 500 mg, Tmax and Cmax are 6 hours and 8 μg/ml respectively, the plasma protein binding rate is 20% to 25%, and the plasma half-life is 1.23 hours. In addition to being unable to pass through the blood-brain barrier, this product can be distributed systemically. 50% of the product is excreted in the urine within 24 hours after oral administration, of which 10% is excreted as metabolites and part of it is excreted in the feces.
Indications[3]
Mainly used for the prevention and treatment of diabetic retinopathy and other microvascular diseases.
Preparation[4]
A synthesis process of calcium dobesilate (monohydrate), which uses hydroquinone and concentrated sulfuric acid to directly sulfonate it, and then neutralizes it with calcium carbonate in an aqueous solution to prepare calcium dobesilate hydrate. . The synthesis process simplifies the operation and saves industrialization costs; the reaction conditions are mild, the product purity is high; the reaction end point is controllable, and it is suitable for industrial large-scale production. This is achieved in the following way: Place concentrated sulfuric acid into a reaction kettle, add hydroquinone at a weight ratio of 1:1 to 1.5 under stirring, and perform a sulfonation reaction, and raise the temperature to 30 to 100 ℃, and maintain the reaction temperature for 1 to 3 hours to obtain a white viscous substance; according to the weight ratio of hydroquinone to purified water is 1:2 to 2.5, add the purified water into the reaction kettle, and slowly add calcium carbonate while stirring. And react until the pH value reaches 2 to 7, stop adding calcium carbonate; centrifuge, and the filtrate obtained is heated and concentrated under stirring. When concentrated to a specific gravity of 1 to 1.5g/ml, the concentrated liquid is cooled and precipitated at room temperature. Crystallize, suction filter, add water to wash to obtain a white crystalline solid crude product, recover the filtrate; mix the crude product and purified water according to the weight ratio of crude product to purified water 1:0.2~10, stir, raise the temperature to 30~100°C, maintain the temperature, and reduce Concentrate under pressure until the specific gravity of the liquid is 1 to 1.8g/ml, hot filter to remove insoluble impurities, crystallize at 0 to 50°C, filter, and wash with purified water 1 to 10 times to make the final filtrate pH 4 -6. Dry to obtain high purity calcium dobesilate (monohydrate).
Main reference materials
[1] CN201510753800.5 Calcium dobesilate tablet and preparation method thereof
[2] CN201410658669.X Calcium dobesilate capsule
[3] Practical medicine hand
[4] Synthesis reaction of CN201010200151.3 calcium dobesilate hydrate

 微信扫一扫打赏
微信扫一扫打赏

