Background and overview[1][2]
Biphenyl-4-sulfonic acid is mainly used in organic synthesis and other fields, such as the preparation of p-phenylphenol. Para-phenylphenol has a wide range of uses. It is mainly used for: preparing heat-sensitive dyes; preparing hot-soluble inks and pressure-sensitive papers for confidential documents; synthesizing extremely durable and weather-resistant resins and coatings; synthesizing hydrophobic fiber accelerators. It can greatly improve the dyeing speed and color fastness; synthesize red and green sensitizing dyes; is widely used in the preparation of new pesticides and medicines with high efficiency and low toxicity; and can synthesize various monomers. In the prior art, biphenyl and sulfuric acid are generally used for sulfonation reaction to prepare biphenyl-4-sulfonic acid, and then sodium hydroxide is added to prepare p-phenylphenol by alkali fusion under high temperature and long reaction conditions. There is also technology to lower the alkali fusion temperature by using potassium hydroxide instead of sodium hydroxide. All these methods produce a large amount of acidic wastewater in the treatment of sulfonated compounds, the purity of the sulfonated compounds is not high, and the reaction temperature during alkali melting is relatively high. Sometimes it is necessary to add high boiling point inert organic medium or water for alkali fusion. The acidic wastewater produced easily pollutes the environment and must be treated before being discharged, which increases production costs.
Structure
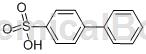
Preparation[1]
Dissolve 31.2 grams (0.2 mol) of biphenyl in 200 ml of o-nitroethylbenzene, control the temperature at 30 to 40°C, slowly add 35 grams of chlorosulfonic acid dropwise, and react at this temperature for 10 to 15 minutes. The reaction mixture was then heated to 80°C for 2 hours. Cool to room temperature, filter, and dry to obtain a dry product weighing 49.2 grams with a biphenyl-4-sulfonic acid content of 98.5%.
Apply[1-2]
Biphenyl-4-sulfonic acid is mainly used in organic synthesis and other fields. Examples of its applications are as follows:
1) Prepare p-phenylphenol. Add 100 ml of biphenyl-4-sulfonic acid and hydrogenated terphenyl, and 167 grams (4 moles) of 96% sodium hydroxide to the Inconel autoclave, and seal the autoclave. Start stirring and raise the temperature to 280°C, keep the reaction for 15 hours, and the pressure rises to 0.8~1.5MPa. After the insulation was completed, the reaction mixture was cooled to 200°C, 300 ml of water was pumped in, the suspension was removed from the autoclave, and the autoclave was rinsed with water. The total volume of the reactants and washing water is 1600 ml. Use a separatory funnel to separate the hydrogenated terphenyl and use it next time. The separated aqueous phase is neutralized with 30% sulfuric acid to a pH value of 4.5 to 6.0. The precipitated product is filtered through a Buchner funnel, washed with clean water, the wet product is dried under vacuum at 100°C, and then purified by sublimation. The product yield is 83% (based on biphenyl), and the purity of p-phenylphenol is 99.9%. The organic solvent filtration product used in the present invention can be recycled for a long time without producing waste water.
2) Preparation of 4-hydroxybiphenyl. Sodium biphenyl-4-sulfonate is reacted with sodium hydroxide at a concentration of approximately 70% by weight to obtain 4-phenylphenolate (approximately 320°C; 16-26 bar), sodium hydroxide and biphenyl-4- The molar ratio of sulfonic acid is approximately 10:1. The reaction mixture was diluted to a 4-hydroxybiphenyl content of approximately 12% by weight by expansion directly into a water bath at 320°C or by pumping water into the reaction mixture after first cooling to 200°C. The resulting alkaline 4-hydroxybiphenyl sodium salt suspension was metered into a water tank, while sulfuric acid was added at a concentration of approximately 50% by weight at 90° C. and pH 5.5. The obtained 4-hydroxybiphenyl containing Na2SO3/NaHSO3 has a 4-hydroxybiphenyl content of approximately 4.3% by weight. The biphenyl suspension is heated at 130-135°C at approximately 2 bar for approximately 2 hours, cooled to 100°C with stirring at a rate of 10 K/h and then cooled to 60°C with stirring. The very easily filterable, coarse crystalline product was separated through a glass sintered filter at 60° C. and washed with water. A virtually colorless, flowable, non-thixotropic, essentially sodium sulfate-free product is obtained, the moisture content of which is significantly reduced to about 10-20% by weight, compared to the 65-70% (without heating according to the invention) obtained. Weight) A highly thixotropic product of water.
Main reference materials
[1] CN200610049123.X Preparation method of p-phenylphenol
[2] Preparation method of CN97113340.94-hydroxybiphenyl

 微信扫一扫打赏
微信扫一扫打赏

