Background and overview[1]
Monomethyl isophthalate can be used as an intermediate for pharmaceutical and chemical synthesis. If monomethyl isophthalate is inhaled, move the patient to fresh air; if skin contact occurs, remove contaminated clothing, rinse skin thoroughly with soap and water, seek medical attention if you feel unwell; if contact with eyes , you should separate your eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse your mouth immediately, do not induce vomiting, and seek medical attention immediately.
Apply[1]
Monomethyl isophthalate can be used as an intermediate for pharmaceutical and chemical synthesis. For example, the following compounds are synthesized:

To a solution of compound monomethyl isophthalate B3 (46g, 0.3mol) in 700mL anhydrous THF, add BH3 in THF (1M, 1M, 600mL, 0.60mol) solution. The solution was then stirred at room temperature overnight. To quench the reaction, 50% aqueous acetic acid (400 mL) was slowly added. The reaction mixture was concentrated and partitioned between EtOAc and water. The organic phase was washed successively with 10% Na2CO3 aqueous solution, H2O and brine. It was dried over Na2SO4, filtered, concentrated under reduced pressure, and purified by silica gel chromatography (eluent: PE/EtOAc = 10/1) to obtain 28 g of compound B4 as a white solid (three-step yield: 54%).
Preparation [1]
The synthesis of monomethyl isophthalate is as follows:
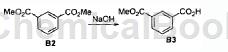
Step 1: Slowly add SOCl 2 (113.3g, 0.96mol) into 700mL of anhydrous methanol at 0°C. After stirring at room temperature for 1 hour, compound isophthalic acid B1 (80g, 0.48mol) was added and stirred for 1.5 hours. The mixture was concentrated, and NaHCO 3 aqueous solution was added for quenching. The suspension was extracted twice with DCM. The combined organic layers were dried with Na 2 SO 4, filtered and concentrated to obtain 85 g of compound dimethyl isophthalate B2, which was used without further purification. Next reaction.

Step 2: To a solution of compound dimethyl isophthalate B2 (60g, 0.31mol) in 500mL MeOH, add a solution of NaOH (12.4g, 0.31mol) in 200mL MeOH. The mixture was stirred at room temperature overnight. It was concentrated, the residue was dissolved in 500 mL of water, and extracted with Et2O. The aqueous solution was acidified with concentrated HCl. In the HCl solution at pH = 2, the white precipitate formed was collected and dried under vacuum to obtain 46 g of crude compound monomethyl isophthalate B3 as a white solid.
Main reference materials
[1] WO201102061.NOVEL FXR (NR1H4 ) BINDING AND ACTIVITY MODULATING COMPOUNDS

 微信扫一扫打赏
微信扫一扫打赏

