Overview[1]
The phenomenon of light emission when an excited state chemical substance produced by a chemical reaction transitions to a lower energy state. When certain substances undergo chemical reactions, they absorb the chemical energy generated during the reaction, causing the electrons in the molecules or atoms to be excited to an excited state. When these excited electrons return to the ground state from the excited state, they emit light radiation. to release energy; or by relying on the energy transfer mechanism, the excitation energy is transferred to the receptor molecule, and then the receptor emits photons. The size and spectral range of light radiation energy are completely determined by the chemical reaction of the substance. Bis(pentafluorophenyl)oxalate is an ester compound that can be used as a chemiluminescent reagent. If bis(pentafluorophenyl) oxalate is inhaled, move the patient to fresh air; if there is skin contact, take off contaminated clothing, wash the skin thoroughly with soap and water, and seek medical treatment if you feel unwell.
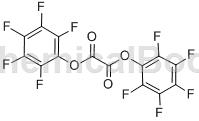
Structure
Preparation method[1]
Bis(pentafluorophenyl) oxalate is prepared as follows: 361.2g (2 moles) of 2,4-dinitrophenol are dissolved in 5 liters of dry benzene, and the dry solution is cooled to10 under nitrogen ℃and add 202.4g (2 moles) of freshly distilled triethylamine. Then chloride (139.6 g; 1.1 mol) was added with stirring at 30°C, minimizing the use of an ice bath to maintain the reaction temperature. The resulting yellow slurry was stirred for 3 hours and evaporated to dryness under reduced pressure. Stir the solid thoroughly, dissolve triethylamine hydrochloride in chloroform, collect the product on a sintered glass funnel, wash with water, and dry under vacuum. The reaction mixture was refluxed for 20 minutes to ensure completion of the reaction, and the product bis(pentafluorophenyl)oxalate was recrystallized from chlorobenzene-diethyl ether (1:1).
Main reference materials
[1] Chemiluminescence from Reactions of Electronegatively Substituted Aryl Oxalates with Hydrogen Peroxide and Fluorescent Compounds’

 微信扫一扫打赏
微信扫一扫打赏

