Background and overview[1]
N1,N1,N4,N4-tetrakis(pyridin-4-yl)benzene-1,4-diamine is an organic intermediate that can be synthesized from 4,4′-bipyridylamine and 1,4-dibromo Benzene is prepared in one step. It is a pyridyl ligand. Pyridine ligands and their derivatives have σ electron-donating ability and π electron-accepting ability, and can form stable complexes with a variety of metals. They are the most widely used chelating ligands in coordination chemistry. These ligands and complexes have been widely used in the fields of molecular catalysis, solar energy conversion, colorimetric analysis, herbicides, molecular recognition, self-assembly, anti-tumor drugs and nucleic acid probes.
Preparation[1]
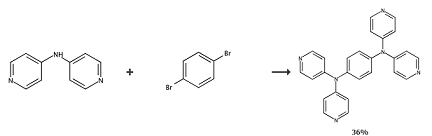
Place 4,4′-bipyridylamine (4.28 g, 25 mmol), 1,4-dibromobenzene (2.36 g, 10 mmol), anhydrous potassium carbonate (4.8 g, 34.4 mmol), copper sulfate ( 994 mg, 6.2 mmol), 18-crown-6 (220 mg, 0.83 mmol) and diphenyl ether (35 mL) were added to the three-necked flask, heated at 200°C under nitrogen for 3 days, and then 1,4- Dibromobenzene (7 g, 29.7 mmol). The reaction mixture was stirred at 200°C for another day. After the reaction was cooled, dichloromethane and water were added to dissolve the solid, and the organic phase was washed with distilled water to neutral pH and then dried over sodium sulfate. After removal of the solvent, the residue was purified by silica gel chromatography to obtain the following two compounds as colorless solids: (4-bromophenyl)-bis(4-pyridyl)amine (2.92 g) and N1,N1,N4,N4 – Tetrakis(pyridin-4-yl)benzene-1,4-diamine (2.1 g, 40.8% based on 4,40-dipyridylamine). 1HNMR (CDCl3, 500MHz): δ = 8.49 (d, J = 6.0Hz, 8H, pyridyl-H), 7.23 (s, 4H, phenylene base-H), 7.01 (d, J = 6.0Hz, 8H, pyridyl-H). Anal. Calcd (%) for C26H20N6: C, 74.98; H, 4.84; N, 20.18. Found: C, 74.57; H, 4.71; N, 20.06. IR (KBr pellet, cm-1 ): 1592(s), 1577 (vs), 1498(s), 1488(s), 130(m), 1304(s), 1277(vs), 1217(m), 993(m), 813(m) ), 740(w), 624(m), 542(m).
Main reference materials
[1] From U.S. Pat. Appl. Publ., 20120214962, 23 Aug 2012

 微信扫一扫打赏
微信扫一扫打赏

