Background and overview[1-2]
5-Amino-2-nitrotrifluorotoluene is an organic intermediate that can be prepared from m-chlorotrifluorotoluene in two steps. There are literature reports that it can be used to prepare pharmaceutical and optical waveguide material intermediates 4-bromo-2-nitrotrifluorotoluene and the non-steroidal anti-androgen drug flutamide.
Preparation[1]
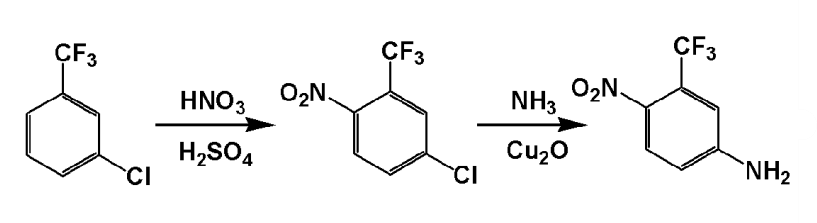
①5‑Chloro‑2‑Nitrotrifluorotoluene
In a 500ml four-neck bottle, add 111.46g (1.15mol) concentrated nitric acid and 402.5g (4.025mo) 98% sulfuric acid in sequence, stir evenly, and add 180.55g (1mol) m-chlorotrifluorotoluene dropwise at room temperature. After the addition is completed, raise the temperature to 50°C and react for 2 hours. Then transfer the nitration liquid to a separatory funnel and let it stand for layering to remove sulfuric acid. The organic phase was washed with 5% sodium carbonate aqueous solution and water in sequence until neutral, and dried to obtain 191g of 5-chloro-2-nitrotrifluorotoluene, with a yield of 84.7%.
Product identification: 1H-NMR (CDCl3, 400MHZ): δ7.92 (1H, d, J=8.8HZ), δ7.82 (1H , d, J=2.0HZ); δ7.75 (1H, dd, J=2.4HZ, J=2.0HZ), m/z=224.98 (100%).
②5-amino-2-nitrotrifluorotoluene
Add 180.8g of 5-chloro-2-nitrotrifluorotoluene obtained in step ①, 566.6g of 24% ammonia water, 34g of liquid ammonia and 9 grams of catalyst into a 1000ml high-pressure reactor, and react at 175°C for 8 hours. The pressure reached 3.6Mpa, lowered to room temperature, and the excess ammonia was removed under reduced pressure. Filtered to obtain 180.8g of 5-amino-2-nitrotrifluorotoluene wet product, which was vacuum dried at 50°C to obtain 155.2g of dry product (Fp.: 127~128℃), the yield is 94%.
Product identification: 1H-NMR (CDCl3, 400MHZ): δ7.99 (1H, d, J=8.8HZ), δ7.00 (1H , d, J=2.4HZ), δ6.80 (1H, dd, J=2.4HZ, J=2.4HZ), δ4.52 (2H, s), m/z=206.03 (100%).
Apply[1-2]
Application 1,
5-Amino-2-nitrotrifluorotoluene can be used to prepare 4-bromo-2-nitrotrifluorotoluene. 4-Bromo-2-Nitrotrifluorotoluene is a very important intermediate for pharmaceutical and optical waveguide materials. 5-Amino-2-nitrotrifluorotoluene is prepared through bromination reaction to obtain 2-nitro-4-bromo-5-aminotrifluorotoluene; then 2-nitro-4-bromo-5-aminotrifluorotoluene is 4-Bromo-2-nitrotrifluorotoluene is prepared through the deamination reaction of tert-butyl nitrite to toluene.
Application 2,
CN201310269675.1 discloses a synthesis process of flutamide, using 5-amino-2-nitrotrifluorotoluene as raw material, 1,2-dichloroethane as solvent, and N,N-dimethyl Acetamide is used as an acid absorbent. Under the action of 4-dimethylaminopyridine, the temperature is maintained at 5~10°C and isobutyryl chloride is added dropwise. After the dropwise addition, the temperature is maintained at 18~25°C to react to 5-amino-2-nitro. The reaction of trifluorotoluene is complete; water is added to raise the temperature to evaporate 1,2-dichloroethane, and the crude product of flutamide is obtained by water separation and filtration; the finished product of flutamide is obtained by recrystallization with ethanol. The process is simple, with few steps, easy to operate, low cost and high product quality. 1) The product conversion rate is high, reaching more than 96%; (2) The catalyst activity is high and the side reactions are small; (3) The reaction conditions are mild and the reaction time is short; (4) The product has high purity and few impurities; (5) Low production cost , the solvent 1,2-dichloroethane can be used repeatedly; (6) There are less three wastes and it is environmentally friendly.
References
[1][China invention, China invention authorization] CN201110424933. Preparation method of X4-bromo-2-nitrotrifluorotoluene
[2][China invention, China invention authorization] Synthesis process of CN201310269675.1 flutamide

 微信扫一扫打赏
微信扫一扫打赏

