Background and overview[1]
4-Cyclohexyl-3-trifluoromethylbenzoic acid is an organic intermediate that can be used to prepare the compound N-(4-cyclohexyl-3-trifluoromethyl-benzyloxy)-ethylimine acid ethyl ester. N-(4-cyclohexyl-3-trifluoromethyl-benzyloxy)-ethyl imide is a synthetic pharmaceutically active compound 1-{4-[1-(4-cyclohexyl-3-trifluoro Intermediate of methyl-benzyloxyimino)-ethyl-benzyl(benxyl)}-azetidine-3-carboxylic acid (“Compound A”). Compound A is a sphingosine-1-phosphate (“S1P”) modulator useful in the treatment of immune diseases such as multiple sclerosis.
Preparation[1]
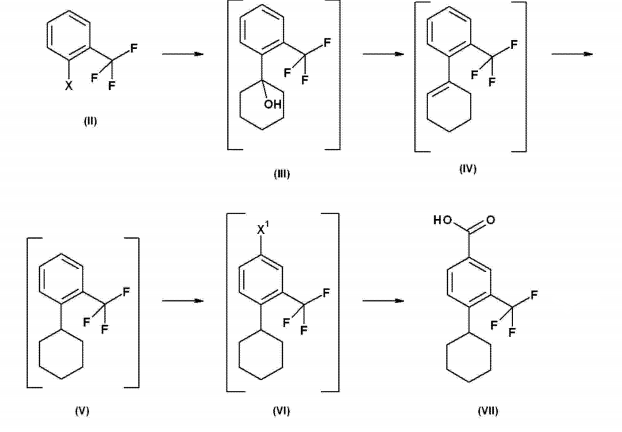
Synthesis of 1-cyclohex-1-enyl-2-trifluoromethyl-benzene
Place isopropyl magnesium chloride-LiCl complex (1.3M) dissolved in 200 ml THF in a dry reactor at room temperature in argon and cool to IT = 5-10°C. Then add 27.5ml of 2-bromotrifluorotoluene within 1 hour (h), keeping IT at 5-10°C. The resulting mixture was stirred at IT=5-10°C for 1 hour. Subsequently, solvent replacement from THF to heptane was performed by distilling off THF while simultaneously adding 120 ml of heptane, keeping the reaction volume constant. To the resulting suspension was added 23.1 ml of cyclohexanone within 1 hour, keeping IT at 15-25°C. The resulting emulsion was stirred at IT=15-25°C for 1-2 hours. After completion, the reaction was quenched by adding 147g H2SO410% at IT=20-30°C. The phases were separated, the aqueous phase was extracted with 14.2 ml of heptane and the combined organic phases were washed with 13.5 ml of water. The organic phase was concentrated to a volume of 120 ml and 42.1g H2SO490% was added within 1 hour, keeping IT at 20-25°C. The resulting mixture was stirred at high speed until the conversion of compound III to compound IV was complete. The phases were then separated and the sulfuric acid phase was extracted with 10 ml of heptane. To the combined organic phases were added 1.46g sodium acetate, 1g silica gel, 1g charcoal and 1ml water. The resulting mixture was filtered on a cellflock-coated suction filter and the filtrate was evaporated to dryness, yielding 35.4 g of 1-cyclohex-1-enyl-2-trifluoromethyl-benzene, which was used for the synthesis of 1- without further purification. Cyclohexyl-2-trifluoromethyl-benzene1H-NMR (400MHz, DMSO-d6): δ1.5-1.7(4H,m),2.1-2.2(4H,m), 5.51(1H,m),7.29(1H,d),7.44(1H,t),7.59(1H,t),7.68(1H,d)MS:(ES-):226(M+).
Synthesis of 1-cyclohexyl-2-trifluoromethyl-benzene
In the hydrogenation reactor, 8.45g of 1-cyclohexyl-2-trifluoromethyl-benzene was dissolved in 42ml of methanol. Add 1.33 g of water-moistened palladium on carbon 10%, and hydrogenate the reaction mixture with hydrogen at IT = 40° C. and 1-5 Bar until hydrogen absorption stops. After filtration on Hyflo, the filtrate was evaporated to dryness and degassed. Heptane (40 ml) was added and the mixture was again evaporated to dryness and degassed. This yielded 7.64 g of 1-cyclohexyl-2-trifluoromethyl-benzene as a slightly turbid yellow oil, which was used without further purification in the synthesis of 4-bromo-1-cyclohexyl-2-trifluoromethyl-benzene. benzene. 1H-NMR (400MHz, CDCl3): δ1.35-1.9(10H,m),2.85-3(1h,m)7.2-7.3(1H, m)7.4-7.5(2h,m),7.55–7.65(1H,m).
Synthesis of 4-bromo-1-cyclohexyl-2-trifluoromethyl-benzene
Dissolve 1-cyclohexyl-2-trifluoromethyl-benzene (38.8g) in 126.4 trifluoroacetic acid at 20-25°C. Then, the solution was cooled to IT=0-5°C and 17.19g H2SO4 (ca96%) was added. To the resulting orange suspension was added 26.73 g of 1,3-dibromo-5,5-dimethylhydantoin in 6 portions at IT=0-5°C within 1-2 hours. 30 minutes after the last addition, perform process quality control and add more 1,3-dibromo-5,5-dimethylhydantoin as needed. After the bromination reaction is complete, 67.5 g of heptane are added and the mixture is stirred for 5-10 minutes (min.) before the phases are separated at IT = 20-25°C. The lower inorganic phase was extracted again with 33.8 ml of heptane. The combined organic phases were extracted with 57.85 g of 10% sodium bisulfite in water, then 55.4 g of 2NNaOH, and 3 times with 41 g of water. Charcoal (0.61 g) was added to the organic phase and the mixture was stirred at room temperature for 1 hour. After filtration, the filtrate was dried by isotropicdistillation and evaporated to dryness. This yielded 49.6 g of 4-bromo-1-cyclohexyl-2-trifluoromethyl-benzene as a yellow oil, which was used for the synthesis of 4-cyclohexyl-3-trifluoromethyl-benzoic acid without further purification. . 1H-NMR (400MHz, DMSO-d6): δ1.2-1.9(10H,m),2.76(1H,T),7.55-7.61(1H,d),7.76.7.85( 2H,m).
Synthesis of 4-cyclohexyl-3-trifluoromethylbenzoic acid
In a dry container, 61.4g of 4-bromo-1-cyclohexyl-2-trifluoromethyl-benzene was dissolved in 230ml of tetrahydrofuran under nitrogen. Isopropylmagnesium chloride (2M, 36.1 ml) dissolved in tetrahydrofuran (THF) was added over 15-30 minutes. Then, the reaction mixture was cooled to IT=-5-+5°C, and 88 ml of 1.6M butyllithium dissolved in hexane was added from the addition funnel within 1-2 hours to keep the IT at -5-+5 ℃. Add 17.6g CO2 to this solution within 1-2 hours at IT=-5-+5°C. After the reaction is completed, quench the reaction by adding 160 ml 2MH2SO4 dropwise, keeping IT at -5-20°C. The phases were separated, the organic phase was washed twice with 100 ml of water and concentrated to a volume of approximately (approximately) 160 ml. Change solvent to toluene. The volume of the toluene solution was then adjusted to approximately 180 ml and heated until a clear solution was obtained. After cooling to 0°C, 4-cyclohexyl-3-trifluoromethyl-benzoic acid crystallized out and was isolated by filtration and then dried in a vacuum oven at 60°C overnight. This yielded 4-cyclohexyl-3-trifluoromethyl-benzoic acid as a white crystalline solid with a purity of >99% (F) by HPLC and a melting point of 206.7-208°C. 1H-NMR (400MHz, DMSO-d6): δ1.2-1.8(10H,m),2.85(1H,m),7.7-8.1(3H,m),13.31(1H,s)MS:(ES-):271(M-1).
References
[1][China invention, China invention authorization] CN201380007968.0 Method for preparing N-(4-cyclohexyl-3-trifluoromethyl-benzyloxy)-ethyl imide [public]

 微信扫一扫打赏
微信扫一扫打赏

