Background and overview[1]
1,3,5-Tris(4-iodophenyl)benzene is an organic intermediate that can be prepared in two steps from 1,3,5-tribromobenzene and p-trimethylsilylbenzeneboronic acid. It has been reported in the literature that 1,3,5-tris(4-iodophenyl)benzene can be used to design and develop high-performance optoelectronic devices, molecular recognition, lithium batteries, hydrogen storage materials and other organic molecular functional materials.
Preparation[1]
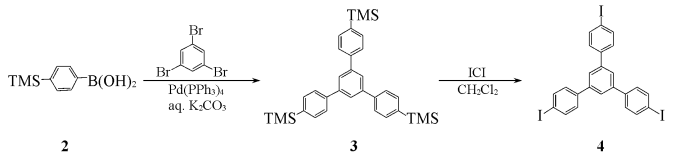
Using deoxytoluene and ethanol (volume ratio 3:1) as a mixed solvent, 1,3,5-tribromobenzene (3.15g, 10mmol), p-trimethylsilylbenzeneboronic acid 2 (7.72g, 40mmol) ) and Pd(PPh3)4(293mg, 0.24mmol)) were added to a 100mL three-necked flask, evacuated, filled with nitrogen, repeated three times, and passed through with a syringe Inject THF (20 mL) and deoxypotassium carbonate solution (2 mol/L, 4.5 mL) into the sealed latex tube, raise the temperature to 85-90°C, and react under nitrogen protection for 48 hours. The crude product is then subjected to column chromatography (dichloromethane as eluent). Lotion) to obtain the intermediate 1,3,5-tris(4′-trimethylsilylphenyl)benzene 3 (3.13g), IR (KBr), ν/cm-1: 2950,1577,1542,1370,1248,1110,836,847,812,752.
Dissolve intermediate 3 in 100 mL of dichloromethane, slowly add iodine chloride (ICl, 4.05 g, 25 mmol) in dichloromethane solution (20 mL) dropwise under nitrogen protection, complete the addition, and react at room temperature for 24 hours. , the reaction solution was washed with a 5% mass fraction of sodium thiosulfate solution, the organic layer was separated, dried with anhydrous MgSO4, concentrated, and column chromatographed (CCl4 (as eluent), recrystallized in carbon tetrachloride to obtain 2.87g of light yellow powder 1,3,5-tris(4-iodophenyl)benzene, yield 42%, m.p.268~271℃.IR ( KBr),ν/cm-1):3033,1593,1483,1437,1377,1243,1061,1003,886,810.1HNMR(CDCl3 ), δ:7.39(dd,6H,J=2.0Hz,J=8.4Hz),7.67(s,3H),7.80(dd,J=2.0Hz,J=8.4Hz,6H).
Apply[1]
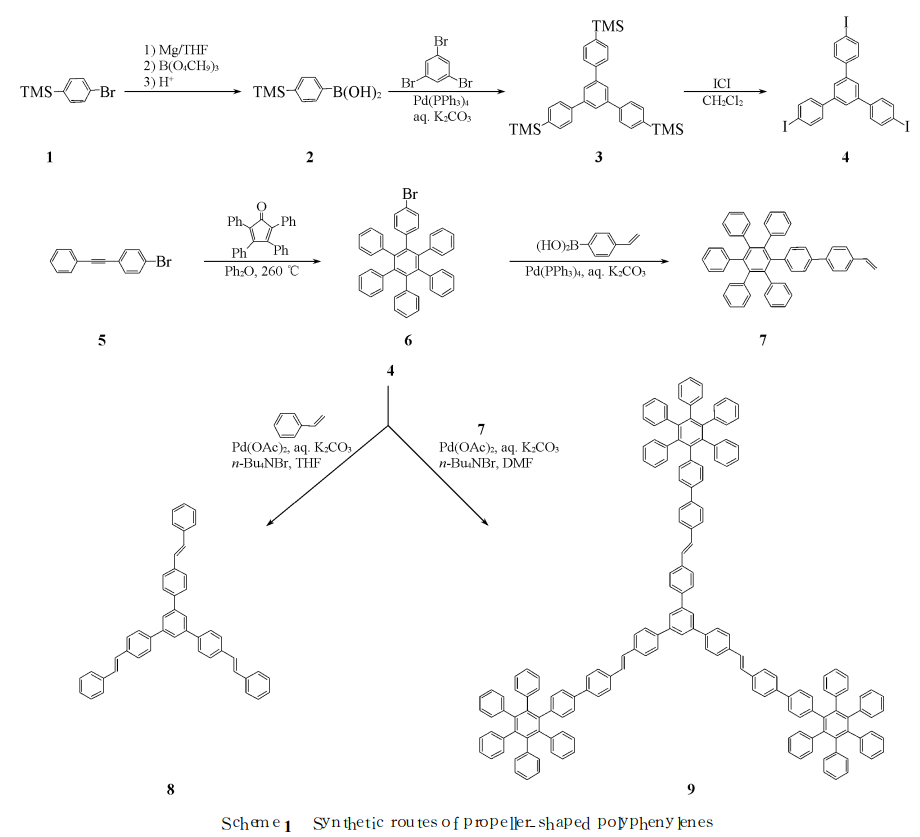
Cheng Ge et al. used 4-vinylbiphenyl as the bridge structure and 1, 3, 5-tris(4-iodophenyl)benzene (4) as the central core through Heck coupling catalyzed by transition metal palladium. The reaction constructs a blue luminescent paddle-shaped molecule (9) containing hexaphenylbenzene structural unit (see Scheme 1 for the synthesis route); for 4-vinylbiphenyl (VBP) as a bridge structure, the “arm” structure of the paddle-shaped molecule In unit 7, the photoluminescence properties of the parent skeleton molecule 1, 3, 5-tris(4-styrylphenyl)benzene (8) and paddle-shaped molecule 9 were studied. Research results show that the paddle-shaped molecule has two luminescent centers, and the maximum emission wavelengths are 397 and 445 nm respectively in the blue light range.
References
[1] Cheng Ge, Zhao Ling, Wang Yuechuan. Synthesis and photoluminescence properties of paddle-type molecules containing hexaphenylbenzene structural units [J]. Journal of Chemistry in Colleges and Universities, 2008(11):2321-2325.

 微信扫一扫打赏
微信扫一扫打赏

