Background and overview[1]
4,5-Difluorobenzene-1,2-diamine is an organic synthesis intermediate and chemical pharmaceutical intermediate. It can be used in the research and development of organic synthesis reaction laboratories and chemical pharmaceutical research and development.
Preparation[1]
Preparation of 4,5-difluorobenzene-1,2-diamine:
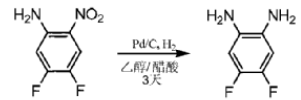
Mix 4,5-difluoro-2-nitroaniline (10g, 57.4mmol), 10% Pdoncarbon (1g) and 100mL methanol together and add it to a reaction bottle filled with nitrogen. Then add 20 mL acetic acid and vent hydrogen gas for 1 minute. The reaction was then left to react at room temperature for 3 days. The Pd catalyst was removed by filtration. The filtrate was collected and distilled under reduced pressure. Then chloroform was added to dissolve the residue. After repeated extraction with saturated Na2CO3 solution three times, it was dried with MgSO4. The organic phase was filtered, the filtrate was collected, and the solvent was evaporated to obtain a brown solid. 1HNMR (400MHz, CDCl3): δ6.51 (t, 2H), 3.33 (br, 4H).
Apply[1]
4,5-Difluorobenzene-1,2-diamine can be used in organic synthesis reactions. If the following reaction occurs:
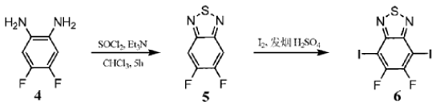
1) Add 0.2g 4,5-difluorobenzene-1,2-diamine (1.38mmol), 20mL chloroform, and 0.57mL triethylamine (5.6mmol) into a round-bottomed flask. Then 0.36g thionyl chloride (2.8mmol) was added dropwise and refluxed for 5 hours. After returning to room temperature, extract with dichloromethane three times, combine the organic phases, dry the organic phases with MgSO4, and evaporate the solvent to obtain 0.2g of white needle-like crystals. 1HNMR (400MHz, CDCl3): δ7.74 (t, 2H, J=8.7Hz).
2) Mix 0.89g compound 5 (5mmol), 5g iodine (, 20mmol) and 25mL fuming sulfuric acid in a round-bottomed flask, and stir at 60°C for 1 day. After the temperature of the reaction system returned to room temperature, the reaction mixture was poured into 300 mL of ice-water mixture. Extract with chloroform three times and combine the organic phases. Then wash the organic phase with saturated NaOH solution and NaHCO3 saturated solution, dry with MgSO4, filter, collect the filtrate, and evaporate the solvent to obtain yellow needle crystals.
Main reference materials
[1]CN103265687-Polymer materials containing bulky branched alkoxy side chains, preparation methods and applications

 微信扫一扫打赏
微信扫一扫打赏

