Background and overview[1][2]
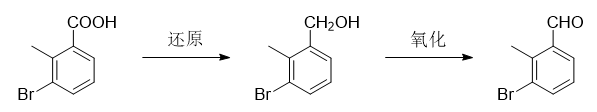
3-Bromo-2-methylbenzaldehyde can be used as a pharmaceutical synthesis intermediate, which can be reduced from 3-bromo-2-methylbenzoic acid as the starting material to obtain (3-bromo-2-methylphenyl ) methanol, and then oxidized to prepare 3-bromo-2-methylbenzaldehyde.
Preparation[1-2]
Report 1:
Step 1: To a suspension of sodium borohydride (0.630 g, 16.7 mmol) in THF (82 mL), add 3-bromo-2-methylbenzoic acid (3.00 g, 14.0 mmol). The mixture was stirred until gas evolution ceased, then iodine (1.77g, 6.98mmol) was added in small portions. The mixture was stirred at room temperature for 2 h, then the reaction was carefully quenched by the slow addition of aqueous HCl (2N). The resulting mixture was diluted with water and extracted with ether. The organic layer was washed three times with aqueous NaOH (2N) and once with brine, dried over magnesium sulfate, filtered and concentrated to afford (3-bromo-2-methylphenyl)methanol (2.79 g) as a white solid.
Step 2: To a solution of dimethyl sulfoxide (2.99 mL, 41.8 mmol) in dichloromethane (55 mL) cooled to -78°C under nitrogen, slowly add oxalyl chloride (2.0 M in dichloromethane) , 10.4mL, 20.8mmol). The mixture was stirred at -78°C for 30 minutes, then a solution of (3-bromo-2-methylphenyl)methanol (2.79 g, 13.9 mmol) in dichloromethane (15 mL) was added dropwise via cannula. The mixture was stirred for 1 hour, triethylamine (5.8 mL, 41.8 mmol) was slowly added, and the reaction was allowed to warm to room temperature. The mixture was washed with saturated aqueous sodium bicarbonate solution, water and brine. The organic layer was dried over magnesium sulfate, filtered and concentrated to give 3-bromo-2-methylbenzaldehyde (2.76g).
Report 2:
Step 1: Dissolve 3-bromo-2-methylbenzoic acid (30g, 139mmol) in tetrahydrofuran (THF) (180mL), cool to 0°C, and add a small amount of lithium aluminum hydride (9.5g, 250mmol) ). After stirring for 3 hours, no starting material was observed by TLC. The reaction mixture was carefully quenched with ethyl acetate (20 mL) and water (20 mL). Add silica gel and evaporate the mixture to dryness and load on a small silica gel column. After evaporation, the product was eluted with hexane:ethyl acetate (1:1) to give pure (3-bromo-2-methylphenyl)methanol (24 g). 1H NMR (600 MHz, CDCl3): 7.50 (d, 1H, J = 8.1Hz), 7.29 (d, 1H, J = 8.1Hz), 7.04 (t, 1H, J = 8.1Hz), 4.68 (s, 2H), 2.40 (s, 3H), 1.90 (br s, 1H).13C NMR (150MHz, CDCl 3): 140.60, 135.84, 132.03, 127.12.
Step 2: Dissolve (3-bromo-2-methylphenyl)methanol (24 g, 119 mmol) in dichloromethane (240 mL) and manganese (IV) oxide (103 g, 1.2 mol). After stirring overnight, TLC showed no starting material. The reaction mixture was evaporated from silica gel and loaded onto a small silica gel column. After evaporation, the product was eluted with hexane:ethyl acetate (10:1) to give the pure aldehyde 3-bromo-2-methylbenzaldehyde (18.6 g). 1 H NMR (600MHz, CDCl3): 10.25 (s, 1H), 7.78 (m, 2H), 7.23 (t, 1H, J = 7.7Hz), 2.75 (s, 3H).13 C NMR (150 MHz, CDCl3): 191.84, 137.72, 130.93, 130.20, 127.61, 127.37, 126.82, 18.13.
Main reference materials
[1] US2018312523A1
[2] WO2016040330

 微信扫一扫打赏
微信扫一扫打赏

