Background and overview[1][2]
(4-Amidophenyl) methanesulfonyl fluoride hydrochloride is a sulfonate derivative that can be used in the preparation of lithium battery electrolyte.
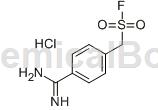
Preparation[1]
A mixture of α-bromo-p-toluonitrile (24.5g; 125mmol) and sodium sulfite (17.0g; 135mmol) in 50ml of water was heated to reflux and refluxed for 18 hours. The mixture, while still hot, was poured into 100 ml of saturated aqueous NaCl solution. The resulting heavy white precipitate was cooled thoroughly, collected by suction filtration, and washed with two parts of saturated aqueous NaCl solution and three parts of Et2O. After vacuum drying over CaSO4, sodium p-cyanophenylmethanesulfonate was obtained in essentially quantitative yield.
In a dry 500 ml round-bottomed flask containing a stirring rod, add anhydrous methanol ( 4.0ml; 3.2g; 100mmol), cool the suspension in an ice bath. Anhydrous HCl was bubbled into the cold suspension in a rapid stream for 90 minutes, and the mixture was stirred at 4°C for 4 days. THF and excess HCl were removed under reduced pressure on a rotary evaporator, leaving a creamy residue. The residue was suspended in anhydrous methanol (150 ml) and the mixture was stirred at room temperature while methanolic ammonia was added dropwise until the mixture became neutral (measured with pH paper). The mixture was heated in an oil bath and maintained at a bath temperature of 60°C-65°C for approximately 3 hours. Check pH occasionally and, if necessary, add additional methanolic ammonia to maintain neutrality. After cooling to room temperature, store the mixture in the refrigerator for two days to allow complete settling. The precipitate was collected by suction filtration and washed with EtOAc. After vacuum drying, the hydrochloride content of crude p-amidophenyl methanesulfonate was 10.7 g (39 mmol; 78%).
In a 100ml round-bottom flask equipped with a stirring rod and equipped with a reflux condenser and a CaCl2 drying tube, prepare dry p-aminophenylmethylsulfonate ammonium salt (10.7g ; 39mmol) and phosphorus oxychloride (50ml) were mixed. The mixture was heated in an oil bath and maintained at a bath temperature of 80°C (±3°C) for 18 hours. After cooling to room temperature, volatile materials were removed under reduced pressure on a rotary evaporator. The residue was mixed with Et2O, and the solid material was collected by suction filtration and washed with Et2O. The solid was dissolved in approximately 250 ml of CH3CN containing 1 ml of POCl3, filtered by gravity, and the filtrate was filtered. Concentrate until a dense precipitate forms. The precipitate was collected by suction filtration, washed with Et2O and dried under vacuum. The yield of p-amidophenylmethylsulfonyl chloride hydrochloride was 4.2 g (15.6 mmol; 31% from sodium p-cyanophenylmethylsulfonate).
In a 50 ml round bottom flask containing a stir bar, prepare sulfonyl chloride (1.0 g; 3.7 mmol) and sodium fluoride (0.5 g; 11.9 mmol) in CH3CN (30 ml) mixture. Install a reflux condenser and a CaCl2 drying tube on the flask, and gently reflux the mixture for 16 hours. After cooling, the mixture was gravity filtered and the filtrate was concentrated to dryness under reduced pressure on a rotary evaporator. Dissolve the solid in about 30 ml of acetone, add 60 ml of 1% HCl aqueous solution, and concentrate the solution under reduced pressure on a rotary evaporator to a small volume. After cooling, a fluffy white needle-shaped product was obtained, which was collected by suction filtration, washed with CH2Cl2 and dried under vacuum. The melting point of p-amidophenylmethylsulfonyl fluoride hydrochloride is 190℃-191℃, which is 0.4g (1.6mmol; 43%).
Apply[2]
In lithium-ion batteries, lithium metal and carbon materials can be used as negative electrode materials. When lithium metal is used as the negative electrode material, it can provide large capacity and high output voltage. The electrolyte currently used is mainly composed of organic solvents and lithium salts. This electrolyte is prone to chemical reactions with lithium metal, causing uneven passivation of the lithium metal surface. As a result, dendrites appear during the charge and discharge process, causing battery failure. Moreover, organic solvents are flammable and explosive, posing safety risks; and the life of conventional lithium batteries is generally 500 charge and discharge cycles, and excellent ones can reach 600 charge and discharge cycles. As an important component of lithium batteries, electrolyte has a direct impact on the life of lithium batteries. Traditional lithium battery electrolytes have large internal resistance, poor stability, and are sensitive to temperature, especially in high-temperature environments.The stability of the solution will decrease sharply, posing a huge safety hazard.
CN201710067174.3 provides an electrolyte for lithium batteries and a preparation method thereof to solve the problems raised in the above background technology. In order to achieve the above object, the present invention provides the following technical solution: the main raw materials in parts by weight of the electrolyte for lithium batteries are: 8 parts of dimethyl sulfoxide, 10 parts of hydroxyurea, 28 parts of cytarabine hydrochloride, poly 7 parts of vinylpyrrolidone, 4 parts of potassium bistrifluoromethanesulfonimide, 2 parts of lithium hexafluorophosphate, 2 parts of 2,4-dichlorophenylhydrazine hydrochloride, 2 parts of trishydroxymethylaminomethane acetate, (4 2 parts of -amidinophenyl)methanesulfonyl fluoride hydrochloride, 0.9 parts of N,N-dimethyl-p-phenylenediamine oxalate.
A method for preparing electrolyte for lithium batteries, the specific steps are:
First, soak lithium hexafluorophosphate, 2,4-dichlorophenylhydrazine hydrochloride and dimethyl sulfoxide in concentrated sulfuric acid solution, then transfer to the reaction kettle and add hydroxyurea and trihydroxymethylaminomethane ester Hydrochloride and (4-amidophenyl) methanesulfonyl fluoride hydrochloride, ultrasonic vibration for 20-30min and incubation at 52-54°C, and finally add cytarabine hydrochloride, polyvinylpyrrolidone, and bistriol every 2 minutes. Potassium fluoromethanesulfonyl imide and N,N-dimethyl p-phenylenediamine oxalate, stir evenly to obtain.
Compared with the existing technology, the beneficial effects of the present invention are:
During the battery charging and discharging process, the electrolyte prepared by the present invention suppresses the capacity attenuation of the battery at low temperatures, and can reduce the amount of gas generated by the electrolyte under high temperature conditions, effectively prevent battery expansion, and improve the high temperature of the battery. performance.
Main reference materials
[1] (US4303592) Amidinophenylmethylsulfonylfluoride
[2] CN201710067174.3 An electrolyte for lithium batteries and its preparation method

 微信扫一扫打赏
微信扫一扫打赏

