Background and overview[1][2]
Cobalt oxalate (CoC2O4), as the main raw material for preparing Co powder, can be used to make cemented carbide. Its structure and properties have a great impact on Co powder. In the preparation of powder materials, the liquid phase precipitation method is a method with simple process, low cost and good powder properties. However, the preparation process conditions of liquid phase precipitation method have a great influence on the properties of powder. Regarding the preparation of cobalt oxalate, there are no reports on the effects of the feeding method, reaction temperature and reactant concentration on the particle size, morphology and yield of cobalt oxalate powder. Research on particle size and morphology is important for broadening the application range of cobalt oxalate. It is very meaningful, and how to improve productivity has real economic value.
Preparation[1]
Using cobalt sulfate, ammonium oxalate, and oxalic acid as raw materials, cobalt oxalate powder is prepared by liquid phase precipitation. The effects of precipitant, feeding method, reaction temperature, reactant concentration, etc. on the particle size and yield of prepared cobalt oxalate powder were studied. The research results show that when ammonium oxalate is used as the precipitant and the reaction temperature is 50°C, the ammonium oxalate solid is quickly added to the cobalt sulfate solution (solid-liquid feeding method), and ultrafine particles with uniform particle size and a particle size of 1.1 μm can be obtained. Cobalt oxalate; when ammonium oxalate solution is added to cobalt sulfate solution (liquid-liquid feeding method), the yield of cobalt oxalate can be as high as 99.6%, but the particle size is larger.
![]()
Both reactions (1) and (2) are performed at 50°C, with the solid precipitant added to a 1.0 mol/L cobalt sulfate solution. In addition, in reaction (2), ammonia water is used to adjust the pH to 2.0. The obtained cobalt oxalates are 1# and 2# respectively. The average particle size of the cobalt oxalate prepared by using ammonium oxalate as the precipitant is smaller, about 1 μm, with a narrow particle size distribution and uniform particle size; while the cobalt oxalate prepared by using oxalic acid as the precipitant is smaller. The average particle size of cobalt oxalate is larger, about 10 μm, and the particle size distribution is wider.
The main reason is: when preparing cobalt oxalate, C2O2-4 is provided by (NH4)2C2O4 solution. Its concentration is high, the precipitation reaction speed is fast, and the number of nucleation per unit time is large. The speed of crystal nucleation and precipitation is also fast, and the particles of CoC2O4 obtained are fine and uniform; while the cobalt oxalate obtained in 2# is slowly ionized due to the slow ionization of H2C2O4 that provides C2O2-4, so C2O2-4 At a lower concentration, CoC2O4 gradually nucleates, the crystal nucleation speed is slow, and the resulting CoC2O4 particles are coarse and uneven.
Apply[2-4]
Cobalt oxalate is used in the preparation of indicators, catalysts and organic synthesis intermediates.
1) Preparation of cobalt tetroxide.
Co3O4 powder is widely used in catalysts, pigments, colored glasses, magnetic materials and ceramics. In the electronics industry, in addition to strict requirements on chemical composition, Co3O4 used in batteries also has special requirements on physical indicators, especially particle size composition, distribution and bulk density. Cobalt oxide is generally required to have a relatively uniform particle size. The typical particle size D50 is 6μm or 10μm, and the bulk density requirement is 0.7~1.2g/cm3. For large batteries used for power supply, the requirements for particle size and bulk density are more stringent due to safety performance considerations. In addition, Co3O4 is also an excellent catalyst material, such as being used as a catalyst for high-temperature catalytic propane combustion. High-purity and ultra-fine Co3O4 is an important raw material for manufacturing heat-sensitive and pressure-sensitive electrodes, color TV glass shells, and high-grade blue and white porcelain. The main oxidative decomposition reactions of cobalt oxalate are as follows:
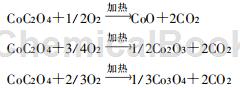
When cobalt oxalate decomposes, the strength and temperature of the oxidizing atmosphere have a great influence on the form of cobalt oxide produced. There are three forms of cobalt oxide, and their grades are CoO78.65%, Co2O371.03%, and Co3O473.43%. According to thermodynamic calculations, when CoO is oxidized in air at 400 to 900°C or in oxygen at 300 to 400°C, Co3O4 can be generated, but only at low temperatures (less than 200°C) or at high temperatures (oxygen pressure exceeds 1.01× Co2O3 can be generated only when 107Pa). Therefore, to obtain the Co3O4 product, the reaction can be controlled in the air at 400 to 900°C.
2) DSC/TG-MS of in-situ thermal decomposition of ammonium perchlorate catalyzed by cobalt oxalate.
Ammonium perchlorate (AP for short) is the most widely used oxidant in solid rocket propellants. Its thermal decomposition characteristics have an important impact on the combustion process of propellants. Cobalt oxalate can strongly catalyze the thermal decomposition of ammonium perchlorate (AP), greatly reducing the decomposition temperature by 104°C. The decomposition reaction speed is very fast and the heat release of the decomposition reaction is very concentrated, with the heat release increasing by 814J/g. Transition metal oxalates show broad application prospects in the field of ammonium perchlorate propellants.
Cobalt oxalate catalyzes ammonium perchlorate in situ. Nanoscale cobalt oxide is directly generated in the catalytic medium. The generated new ecological catalyst has high catalytic activity, large specific surface area, and participates in the catalytic reaction immediately, maximizing the use of nanometer-sized cobalt oxide. Catalyst efficiency. The increase in the apparent reaction heat effect of ammonium perchlorate decomposition is mainly due to the adsorption of oxygen on the surface of the new ecological cobalt oxide to generate oxygen peroxide ions (O-2), thereby accelerating the oxidation of ammonia adsorbed on the active center. .
3) Prepare a large particle size cobalt powder.
The preparation method of large particle size cobalt powder includes the following steps: providing a reaction bottom liquid; adding ammonium oxalate and cobalt salt solution to the reaction bottom liquid, reacting at 75~95°C to generate cobalt oxalate precipitate, and wait for When the D50 particle size of the cobalt oxalate reaches 60 μm, the reaction is stopped and aging treatment is performed; the aged cobalt oxalate is subjected to solid-liquid separation, drying, and crushing to obtain a cobalt oxalate precursor; the cobalt oxalate is The precursor is reduced and crushed to obtain the large particle size cobalt powder.
The preparation method of large particle size cobalt powder is simple in process, easy to control, low in cost and suitable for industrial production. The cobalt powder obtained by this large particle size cobalt powder preparation method has high purity, good dispersion, and uniform particle size. The D50 particle size is relatively large, and the D50 particle size is 20~30 μm, which meets the diversified demand for cobalt powder in the industry. .
Main reference materials
[1] Research on preparation of cobalt oxalate powder by liquid precipitation method
[2] Preparation of Co3O4 from thermal decomposition of cobalt oxalate and characterization of its physical properties
[3] DSC/TG-MS study on in-situ catalytic thermal decomposition of ammonium perchlorate by cobalt oxalate
[4] CN201110212720.0 A large particle size cobalt powder and its preparation method

 微信扫一扫打赏
微信扫一扫打赏

